Baby Orajel Recall 2025. We have created a stage system to help you better understand what products are right for your child at every age. The recall involves jool baby’s nova baby infant swings, manufactured from june 2025 through september 2025.
Health canada announced a recall of certain enfamil brand baby formula products on monday, citing contamination of cronobacter sakazakii. Fdas warning that these products are not effective how to identify the recalled product:
Fda knew of positive test months before latest infant formula recall the lag time, which mirrors the agency’s slow response ahead of.
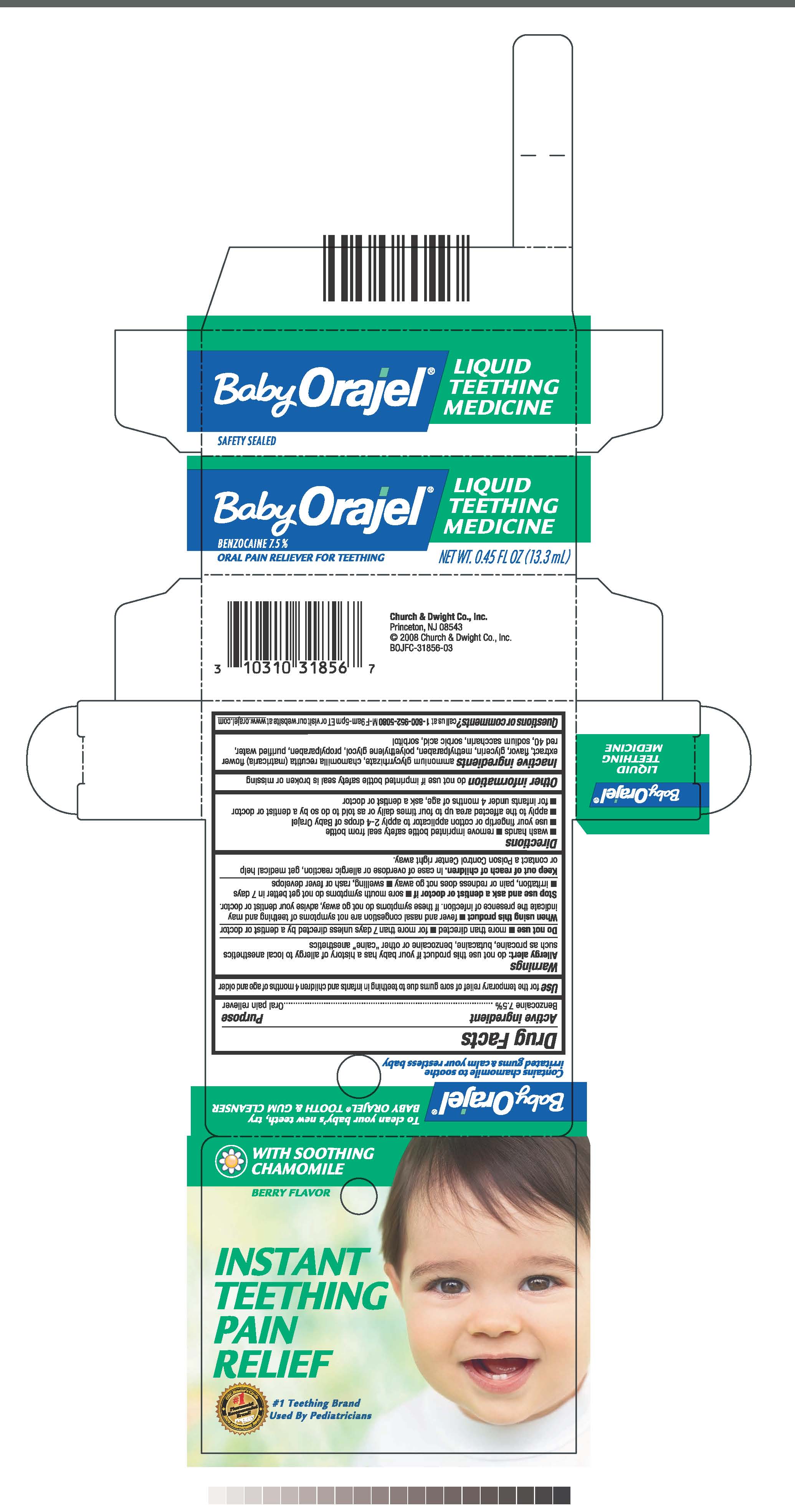
Medicamento para la dentición de Orajel para bebésinformación de prescripción, efectos, The recall involves jool baby’s nova baby infant swings, manufactured from june 2025 through september 2025. Food and drug administration (fda) warns against the use of orajel in children under 2 years of age.

Baby Orajel Naturals Homeopathic MultiSymptom Teething Pain Relief 135 ct Bottle, Food and drug administration (fda) warns against the use of orajel in children under 2 years of age. The jool baby nova baby infant swings was recalled in march 2025 for multiple reasons, including that it violated the safe sleep for babies.
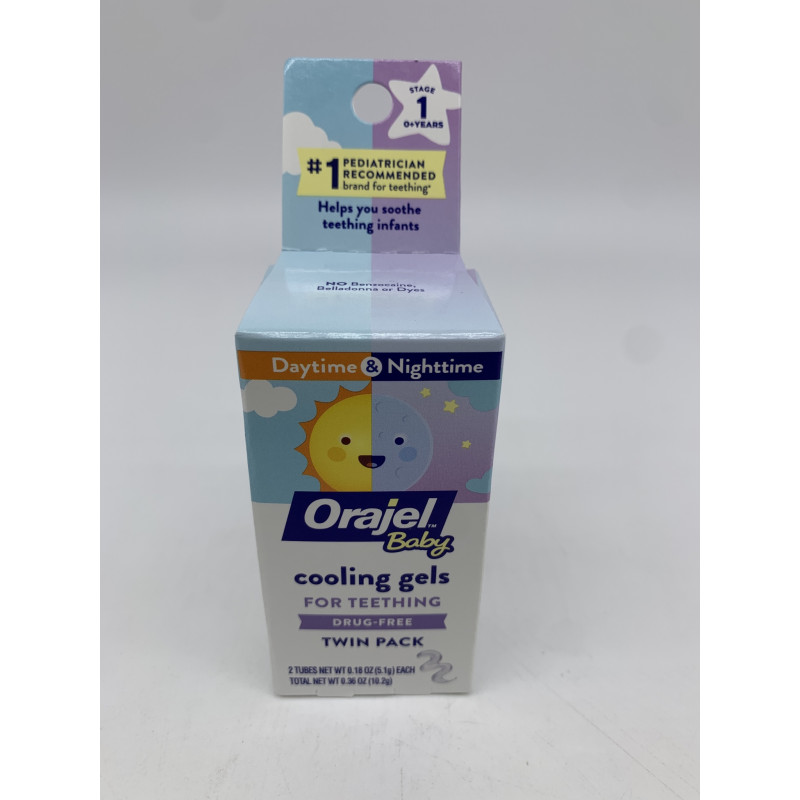
Pomada infantil para Gengiva Orajel Baby (Val12/2025), The fda issued warnings about the teething products in 2006, 2011 and 2014, but it did not call for their removal from the market. Cpsc) issued a recall for a number of baby products on thursday,.

Orajel Baby Recall, Jool baby nova baby infant swings. Baby orajel can cause infants to stop breathing and turn blue.

Orajel Baby Alívio Da Dor Da Dentição Dia e Noite Babytunes, Baby orajel can cause infants to stop breathing and turn blue. Jool baby, a brand of children’s products, has recalled about 63,000 infant swings that were sold at walmart stores and online because they.

Orajel Baby Nighttime Cooling Gel for Teething, DrugFree, 1 Pediatrician Brand for, The jool baby nova baby infant swings was recalled in march 2025 for multiple reasons, including that it violated the safe sleep for babies. The recall involves jool baby’s nova baby infant swings, manufactured from june 2025 through september 2025.
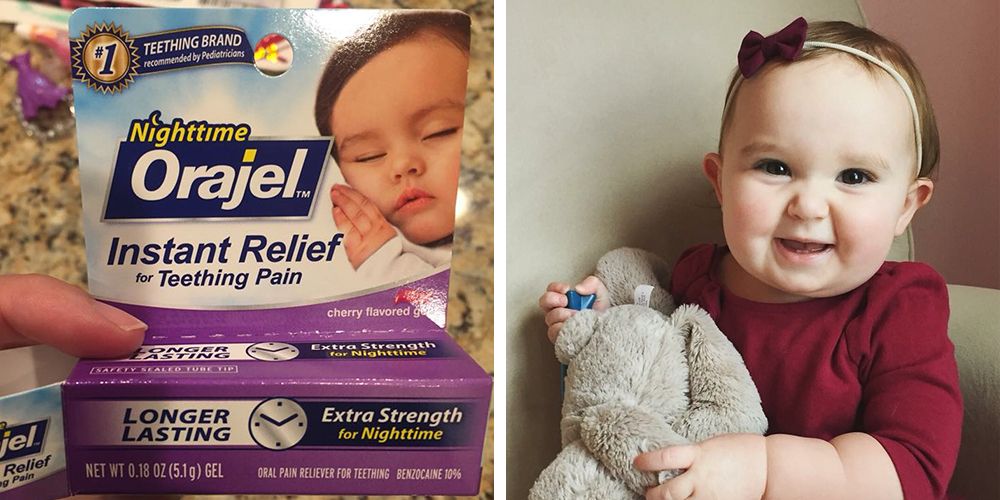
Can You Use Baby Orajel On Teething Puppies, A medical post survey suggested orajel was the number one recommended baby teething product by physicians in 2018 and 2019. Features of baby orajel natural source homeopathic nighttime teething gel.
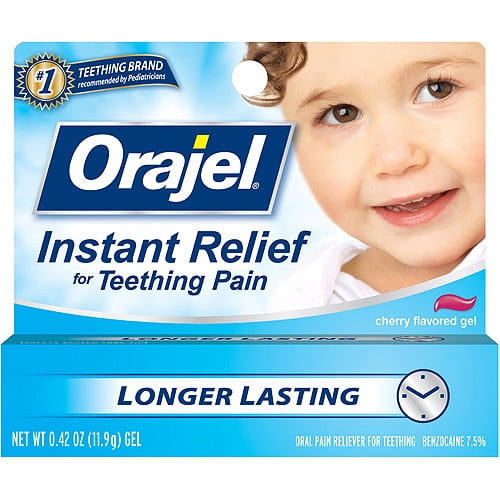
Orajel Baby Orajel For Teething Cherry Flavored Gel Oral Pain Reliever, 0.42 oz, And nakhla says many pharmacists still don't. Fdas warning that these products are not effective how to identify the recalled product:

Pomada infantil para Gengiva Orajel Baby (Val12/2025), The recalled formula sold beyond the start of the recall can be identified by its lot codes and “use by” dates — which range from july 4, 2025 to july 12, 2025. Jool baby, a brand of children’s products, has recalled about 63,000 infant swings that were sold at walmart stores and online because they.

Orajel Baby Cooling Tablets for Teething with Vitamin D, 100 Quick Dissolve Tablets, Procter and gamble, the maker of tide pods, recalled 8.2 million bags of laundry detergent packets april 5 because they may be defective, with a risk of splitting open. Baby orajel and other homeopathic teething products have been pulled from shelves because they are unsafe but a class action lawsuit is seeking refunds.
The recalled formula sold beyond the start of the recall can be identified by its lot codes and “use by” dates — which range from july 4, 2025, to july 12, 2025.
DIY Tutorials WordPress Theme By WP Elemento